LC-SMCC crosslinker
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| PCL026_0100 | 0,1 g | 205,00 € | in stock | |
| PCL026_0250 | 0,25 g | 450,00 € | in stock | |
| PCL026_1 | 1 g | 1.600,00 € | in stock |
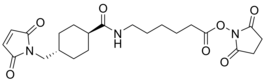
- IUPAC Name: 2,5-Dioxopyrrolidin-1-yl 6-(4-((2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)methyl)cyclohexane-1-carboxamido)hexanoate
- Synonyms: 2,5-dioxopyrrolidin-1-yl 6-(4-((2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)methyl)cyclohexane-1-carboxamido)hexanoate, succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxy-(6-amidocaproate), N-succinimidyl 6-[[4-(maleimidomethyl)cyclohexyl]carboxamido]caproate, N-succinimidyl 4-(N-maleinimidomethyl)-cyclohexane-1-carboxy-(6-amidocaproate), succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxy-[6-amidocaproate], LC-SMCC
- CAS: 1337538-59-6
- Smiles: O=C(CCC1=O)N1OC(CCCCCNC(C2CCC(CN3C(C=CC3=O)=O)CC2)=O)=O
- Chemical formula: C22H29N3O7
- Molecular weight: 447.488
- Purity: 95%+
LC-SMCC is a heterobifunctional crosslinker with N-hydroxysuccinimide (NHS) ester and maleimide group at the opposite ends of the molecule. This allows the conjugation of both amine and sulfohydryl-containing molecules. NHS esters react with primary amines at pH 7-9 to form amide bonds, while the maleimides react with sulfhydryl groups at pH 6.5-7.5 to form stable thioether bonds. In aqueous solutions, hydrolytic degradation of NHS is a competing reaction and its rate is increased by higher pH. Maleimide group is on the other hand more stable than NHS but can slowly hydrolyze and lose its reaction specificity towards sulfhydryls above the pH of 7.5. For the above-mentioned reasons, conjugation is advised to be done at pH 7,2-7,5 while the NHS-ester reaction should be done simultaneously or before the reaction of maleimide.
