5-Azido-1,1,1-trifluoropentan-2-one
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| FAZ019_0050 | 0.05 g | 850,00 € | in stock | |
| FAZ019_0100 | 0.1 g | 1.350,00 € | in stock | |
| FAZ019_0250 | 0.25 g | 2.750,00 € | in stock |
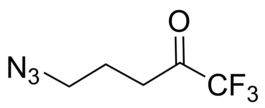
- IUPAC Name: 5-Azido-1,1,1-trifluoropentan-2-one
- Synonyms:
- CAS: N/A
- Smiles: O=C(CCCN=[N+]=[N-])C(F)(F)F
- Chemical formula: C5H6F3N3O
- Molecular weight: 181.12
- Purity: 95%+
This azidoalkyl trifluoromethyl ketone introduces a highly electrophilic trifluoromethyl ketone functionality by click chemistry.
Trifluoromethyl ketones can be used for multiple purposes:
- They may be used per se as a part of a drug candidate and act as an electrophilic warhead, reversibly forming adducts with various nucleophiles, such as water, oxygen-, nitrogen- and sulfur-centered nucleophiles derived from amino acid side chains. Trifluoromethyl ketones can be used for design of new serine protease inhibitors1-3 (through hemiacetal forming interference with oxyanion hole), kinase inhibitors,4 SARS-CoV 3CL protease5 or act as a "molecular glue".
- As the trifluoromethyl ketone is highly hydrated, the gem-diol may act as a zinc-binding group and be used as an integral part of metalloenzyme inhibitors, such as histone-deacetylase.6,7
- They can be transformed to a variety of different derivatives, for example they can be easily reduced to secondary trifluoromethylated alcohols, undergo Friedel-Crafts reaction with electron rich aromatics such as indoles, participate in tandem imine formation followed by hydride reduction to afford α-trifluoromethylated amines or add carbon-centered nucleophiles such as acetylenes.
When attached to an alkynylated biomolecule (protein, peptide, nucleic acid, glycan, etc.) or advanced material, the 3 magnetically equivalent fluorines can act as 19F NMR sensor of its surroundings, for example to detect the local degree of hydration or reversibly form adducts with neighbouring amino acid side chains.
References:
1) Med. Chem. Commun., 2018, 9, 1011-1016.
2) Molecules, 2020, 25 (17), 4031.
3) BMC Mol and Cell Biol, 2020, 21, 38.
4) Bioorg. Med. Chem., 2021, 50, 116457.
5) Bioorg. Med. Chem., 2008, 16, 4652-4660.
6) Med. Chem. Commun., 2016, 7, 464-470.
7) Synthesis, 2018, 50 (20), 4037-4046.
